Written by Rohan Narayanan on May 11, 2022
Washington, DC — The National Organization for Rare Disorders (NORD) applauds efforts of Congressional leaders to protect the Orphan Drug Act (ODA) and ensure proper incentives are in place to continue to foster robust rare disease drug development. Last week, leaders of the House Energy and Commerce Committee… Read More
Written by Lisa Sencen on May 7, 2019

Washington, DC, May 7, 2019 – The National Organization for Rare Disorders (NORD)Ⓡ, the leading independent nonprofit organization representing the 25-30 million Americans living with rare diseases, has announced its development of key drug pricing principles, created with the needs of the rare disease community in mind.
People living with rare… Read More
Written by Lisa Sencen on March 18, 2019
Today, Representatives GK Butterfield and Gus Bilirakis introduced a resolution that celebrates the success of the Orphan Drug Act (ODA) and calls for continued support of the legislation.
Now in its 36th year, the ODA has successfully encouraged the pharmaceutical industry to develop therapies for those with rare diseases. Prior to… Read More
Written by Lisa Sencen on November 19, 2018
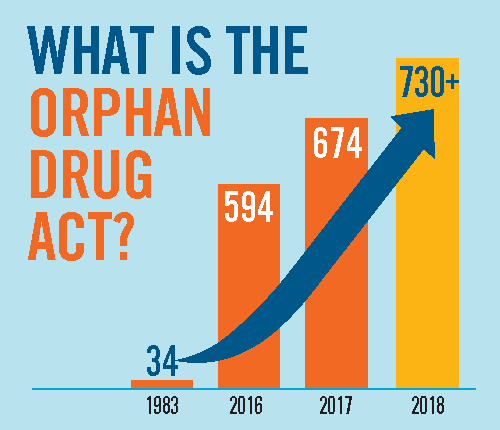
Senator Hatch and Representatives Lance and Butterfield introduced a resolution that heralds the success of the Orphan Drug Act (ODA) and calls for continued support of the legislation.
This year marks the 35th anniversary for the ODA. That means 35 years of increased hope and treatments for the rare disease community…. Read More
Written by Jennifer Huron on July 30, 2015
Washington, D.C., July 30, 2015 – The National Organization for Rare Disorders (NORD) has filed an amicus brief in the D.C. Circuit of the U.S. Court of Appeals, stating that a recent Food and Drug Administration (FDA) decision has the potential to impede the development of new treatments to… Read More








